An Unexpected Legacy in Our Blood
In a quiet bone lab in Copenhagen, researchers have traced a modern genetic shield against HIV to a single ancient ancestor—someone who lived thousands of years ago, near the shores of the Black Sea. The story of this mutation is not one of modern medicine alone. It's a story of migration, survival, and the biological cost of living together in large, sedentary communities.
Known as the CCR5-Δ32 deletion, this genetic variant disrupts a receptor used by HIV to enter human cells. About one in five people in Denmark carry it. Some are resistant to HIV infection; a smaller number are functionally immune. But this mutation did not emerge in response to HIV, which only appeared in humans in the 20th century.
So why does this mutation exist at all?
A Mutation Born of Crisis
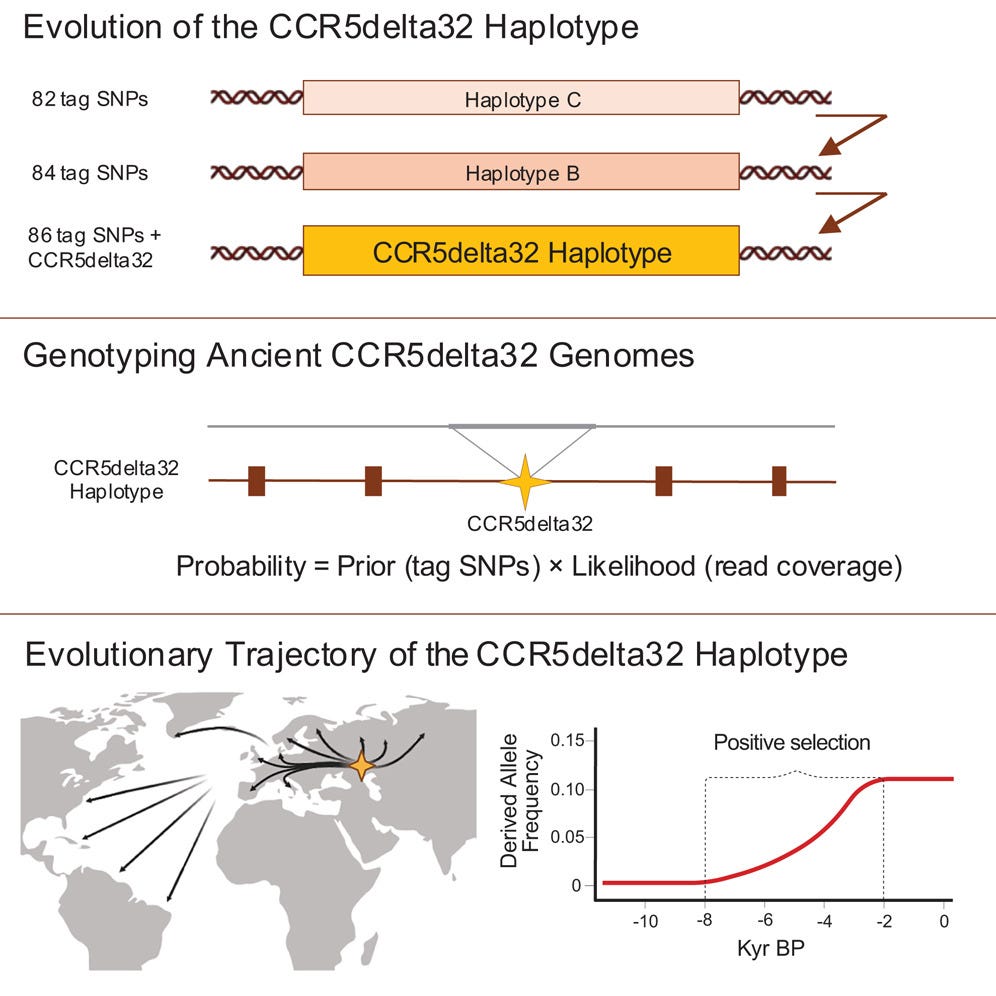
The new study, published in Cell1, maps the origin and spread of the CCR5-Δ32 deletion using a combination of modern genomes and ancient DNA. The mutation, researchers report, first appears in an individual who lived between 6,700 and 9,000 years ago in the vicinity of the Black Sea.
“By looking at this large dataset, we can determine where and when the mutation arose,” said Kirstine Ravn, a senior researcher at the Novo Nordisk Foundation Center for Basic Metabolic Research.
The team analyzed genetic material from over 2,000 living individuals alongside 900 ancient genomes ranging from the early Neolithic to the Viking Age. By developing a new AI-assisted technique to detect CCR5 deletions in degraded DNA, they spotted a sudden, rapid rise of the mutation beginning roughly 5,000 years ago.
Its spread was swift—too swift for genetic drift alone.
“People with this mutation were better at surviving,” said postdoctoral researcher Leonardo Cobuccio. “It probably dampened the immune response at a time when humans were encountering new pathogens.”
Agriculture, Crowds, and Contagion
The mutation’s timing coincides with one of the most significant transitions in human history: the rise of agriculture. As populations shifted from mobile foragers to settled farmers, their daily lives changed dramatically. Grain storage brought mice. Animal domestication brought zoonoses. Sedentism brought waste, and with it, disease.
In such conditions, a hyperactive immune system could become a liability. The CCR5 deletion may have offered a buffer. By slightly muting immune responses, it may have reduced mortality during early exposure to smallpox-like viruses or other epidemic pathogens that flourished in these dense, early farming settlements.
“An overly aggressive immune system can be deadly—think of cytokine storms in severe flu or COVID-19,” Cobuccio added.
In that light, the deletion may have helped human groups adjust biologically to a radically new way of living.
A Genetic Ghost, Reanimated by HIV
Fast forward to the 1980s, and physicians begin to notice something odd. A small subset of people exposed to HIV remained uninfected. Eventually, scientists identified the CCR5-Δ32 mutation as a likely cause. Today, the variant plays a central role in therapies and even stem cell transplants designed to treat or cure HIV.
What makes this story compelling isn’t just the age of the mutation—it’s the irony. A genetic variation born in the context of Neolithic plagues now protects people against a disease that didn't exist until the late 20th century. It’s as if an ancient ghost in our genome, shaped by the first waves of crowd-borne diseases, returned to protect its descendants.
A Broader Story of Human Evolution
This research is the latest in a growing body of studies that link ancient evolutionary pressures to modern health. In recent years, ancient DNA has been used to track how populations responded to the Black Death, tuberculosis, and leprosy—all diseases that shaped the human genome.
The story of CCR5-Δ32 is a case study in evolutionary contingency. A single deletion in one individual's genome became a tool for modern survival. But it also hints at the costs of civilization itself—the diseases we created, the biological changes they forced, and the delicate balance between immunity and inflammation.
Related Research
Fumagalli, M., Sironi, M., Pozzoli, U., Ferrer-Admetlla, A., Pattini, L., & Nielsen, R. (2011). Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genetics, 7(11), e1002355. https://doi.org/10.1371/journal.pgen.1002355
Enard, D., Cai, L., Gwennap, C., & Petrov, D. A. (2016). Viruses are a dominant driver of protein adaptation in mammals. eLife, 5, e12469. https://doi.org/10.7554/eLife.12469
Souilmi, Y., Patin, E., Sousa, V. C., et al. (2021). Ancient viral epidemics and the evolution of human adaptive immunity. PLOS Genetics, 17(6), e1009772. https://doi.org/10.1371/journal.pgen.1009772
Ravn, K., Cobuccio, L., Muktupavela, R. A., Meisner, J., Danielsen, L. S., Benros, M. E., Korneliussen, T. S., Sikora, M., Willerslev, E., Allentoft, M. E., Irving-Pease, E. K., & Rasmussen, S. (2025). Tracing the evolutionary history of the CCR5delta32 deletion via ancient and modern genomes. Cell. https://doi.org/10.1016/j.cell.2025.04.015