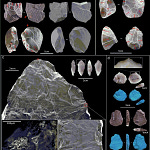
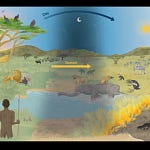
The Tropical Puzzle in Our DNA
Long before Homo sapiens left Africa, another hominin was roaming Eurasia. Denisovans, an extinct branch of our genus, inhabited a mosaic of landscapes stretching from the Siberian taiga to the subtropical forests of Southeast Asia. Their genes persist in modern humans, especially in Melanesia and Southeast Asia, where traces of Denisovan ancestry rise above 5 percent. But why do these archaic alleles persist — and why are they linked to immunity?
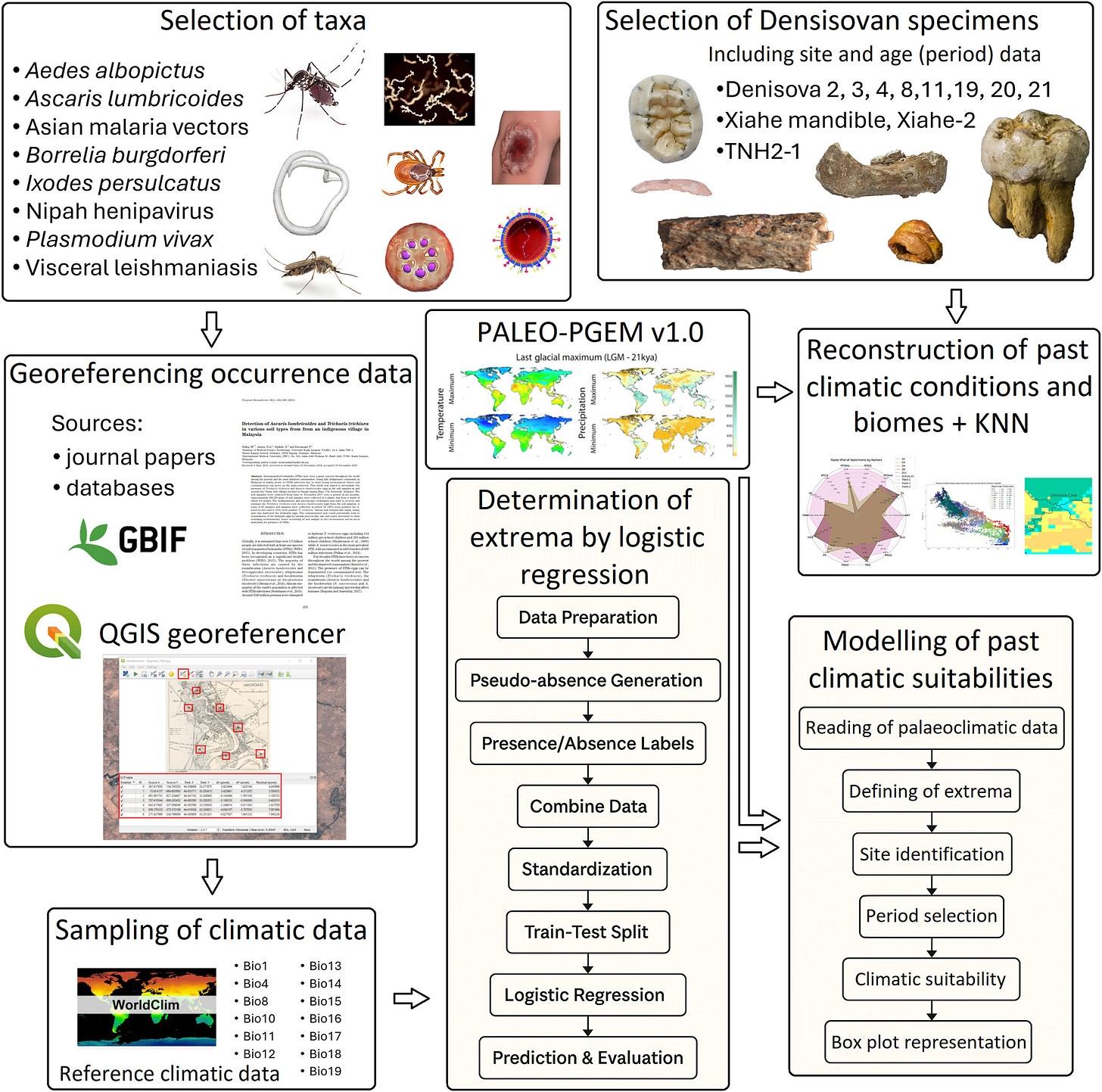
A new study by Attila Trájer in the Journal of Human Evolution1 takes a different approach to this question: instead of focusing only on the genes, it reconstructs the environments Denisovans lived in, then compares them to the immune-related DNA we still carry.
“The Denisovan genetic legacy is particularly high among present-day Melanesians and some Indigenous Philippine groups. Their ancient habitats may explain why we see certain immune alleles in these populations today,” writes Trájer.
Mapping the Paleohabitats
The study models three confirmed Denisovan sites: Denisova Cave in Siberia, Baishiya Karst Cave on the Tibetan Plateau, and Tam Ngu Hao 2 (“Cobra Cave”) in Laos. Each site tells a different ecological story.
Denisova Cave: Subarctic boreal forest, seasonal extremes, and tick-borne disease risk.
Baishiya Karst Cave: High-altitude monsoon-influenced subarctic environment, cold but with pulses of moisture.
Tam Ngu Hao 2 Cave: Humid subtropical setting with monsoon rains, likely rich in mosquitoes, helminths, and other parasites.
“The Cobra Cave site stands out as a tropical or subtropical environment with optimal conditions for disease transmission,” Trájer reports.
By modeling paleoclimate and known ranges of eight disease vectors (including Plasmodium vivax, Ixodes ticks, and Aedes albopictus mosquitoes), Trájer shows that Denisovan populations were likely exposed to radically different pathogen communities depending on geography. Siberian Denisovans contended with cold-weather zoonoses such as tick-borne encephalitis and Lyme borreliosis, while the Laotian Denisovans faced malaria, helminths, and Nipah-like viruses.
Ancient Immunity, Modern Consequences
The research highlights specific Denisovan-derived immune alleles such as HLA-H*02:07 and toll-like receptor variants still present in modern populations. These genes shape how the body recognizes viruses, bacteria, and parasites.
“Denisovan habitats shaped modern human disease resistance,” Trájer writes, noting that introgressed alleles may have balanced pathogen defense with autoimmune risks.
Populations in Melanesia today exhibit high frequencies of these alleles, yet live in regions without the cold-weather pathogens of Siberia. This suggests the inherited alleles may have been selected in tropical or monsoon climates — precisely the conditions reconstructed at the Cobra Cave site.
Why This Matters for Anthropologists and Archaeologists
This approach bridges two fields often kept apart: paleoecology and immunogenetics. Instead of treating Denisovans as a static genome donor, it reframes them as living organisms responding to disease landscapes — much like hunter-gatherers today. It also deepens debates over the timing and routes of archaic-human dispersals into Southeast Asia.
The study further suggests that Denisovan cytochrome P450 gene variants, known for metabolizing plant and animal toxins, may have helped them survive in biodiverse environments teeming with venomous snakes, poisonous plants, and mosquito-borne parasites.
Rethinking Human Evolution as an Immunological Process
For archaeologists, the implications are clear: sites are not just dots on a map. They’re embedded in ecosystems that left molecular fingerprints in our DNA. Future excavations at Southeast Asian caves may reveal not only stone tools or teeth but also isotopic signatures and microfossils of pathogens, shedding light on the coevolution of hominins and disease.
“The Denisovan and shared archaic heritage of these alleles highlights how ancient gene variants continue to shape the modern immune landscape,” Trájer notes.
Related Research
Vernot et al. (2016). “Excavating Neandertal and Denisovan DNA from the genomes of Melanesians.” Science. https://doi.org/10.1126/science.aad9416
Larena et al. (2021). “Multiple deeply divergent Denisovan ancestries in Papuans.” Nature Ecology & Evolution. https://doi.org/10.1038/s41559-020-1127-1
Dannemann, M., & Kelso, J. (2017). “The contribution of Neanderthals to phenotypic variation in modern humans.” American Journal of Human Genetics. https://doi.org/10.1016/j.ajhg.2017.09.001
Hubert et al. (2022). “Denisovan introgression and immune gene adaptation in modern humans.” PNAS. https://doi.org/10.1073/pnas.2102859119
Trájer, A. J. (2025). The role of Denisovan paleohabitats in shaping modern human genetic resistance to viral, bacterial, and parasitic infections. Journal of Human Evolution, 207(103746), 103746. https://doi.org/10.1016/j.jhevol.2025.103746