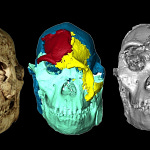
What makes Homo sapiens tall and metabolically demanding compared to our primate cousins? Scientists have long attributed these traits to changes in diet, lifestyle, and brain size, but recent work1 suggests a deeper molecular story—one that begins with a single mutation, likely more than half a million years old.
A Mutation in the Mitochondria's Metabolic Machinery
In a study published in Cell Genomics, researchers identified a regulatory genetic variant known as rs34590044-A that appears to have co-influenced both height and basal metabolic rate (BMR) in anatomically modern humans. The variant boosts the activity of a mitochondrial gene, ACSF3, which is involved in breaking down essential amino acids, particularly threonine—a key component of meat-heavy diets.
"The human-specific rs34590044-A allele can upregulate the expression of ACSF3 compared to the ancestral C allele," the authors reported, adding that this enhanced expression helps avoid buildup of the toxic metabolite methylmalonic acid (MMA), which can impair mitochondrial function.
This small change in the genome, likely under positive selection for the past 20,000 years, has been tied to two seemingly unrelated human traits: greater height and increased baseline energy expenditure.
Energy Demands of a Taller Species
Compared to our closest relatives—chimpanzees, bonobos, and gorillas—Homo sapiens are about 30 centimeters taller and have a BMR up to 50% higher, even when adjusted for body mass. The metabolic implications are enormous. Taller bodies require more energy not just for growth but also for thermoregulation and maintenance.
The research team analyzed genetic data from over 450,000 individuals in the UK Biobank and found a strong genetic correlation between BMR and height. They identified more than 6,000 genetic variants likely influencing both traits. Among them, the ACSF3 variant stood out.
Laboratory experiments using mouse models and human cell lines revealed that increased expression of ACSF3 improved mitochondrial activity, especially when animals were fed a threonine-rich diet. This not only enhanced energy production but also supported bone growth—suggesting that the metabolic and skeletal traits of modern humans are intertwined at the molecular level.
Ancient Diets, Modern Physiology
The evolutionary story doesn’t stop at genes. The ACSF3 mutation likely gained an advantage in humans consuming more animal protein. Essential amino acids like threonine are scarce in plant-based foods but plentiful in meat. Enhanced processing of these amino acids would have supported the energy-intensive physiology of a species with large brains, long legs, and high reproductive costs.
"Our findings support the idea that ACSF3 functions as a molecular bridge between nutrition and the emergence of key human traits," the study notes.
Data from ancient genomes back this up. The frequency of the rs34590044-A variant surged in European populations during the past 5,000 years, coinciding with major shifts in diet and mobility brought by pastoralist groups like the Yamnaya. The allele is now common in Europe, particularly in Britain.
A Window Into Our Adaptive Past
The story of ACSF3 shows how evolution often works through small molecular tweaks with big downstream effects. By enabling efficient metabolism of protein-rich foods, this single variant may have helped early humans grow taller, sustain larger brains, and thrive in new environments.
This research adds to a growing list of genetic changes shaped by dietary transitions: lactase persistence for digesting milk, amylase copy number variation for starch-rich diets, and FADS1 variants influencing fatty acid synthesis in farming populations. Together, they map out a genomic record of how food has sculpted the human body.
"The evolutionary history of Homo sapiens is as much a story of what we ate as where we walked."
Related Research
Llamas, B., et al. (2021). From the cradle of humanity to the edge of the world: Ancient genomics and the prehistoric dispersal to Oceania. Nature, 592, 583–589. https://doi.org/10.1038/s41586-021-03236-5
Perry, G. H., et al. (2007). Diet and the evolution of human amylase gene copy number variation. Nature Genetics, 39(10), 1256–1260. https://doi.org/10.1038/ng2123
Mathieson, I., et al. (2015). Genome-wide patterns of selection in 230 ancient Eurasians. Nature, 528, 499–503. https://doi.org/10.1038/nature16152
Enard, W., & Paabo, S. (2004). Comparative primate genomics and the evolution of human biology. Science, 306(5699), 1120–1123. https://doi.org/10.1126/science.1103936
Fan, S., et al. (2025). An ancient regulatory variant of ACSF3 influences the coevolution of increased human height and basal metabolic rate via metabolic homeostasis. Cell Genomics, 5, 100855. https://doi.org/10.1016/j.xgen.2025.100855
Zhang, Y., Wang, J., Yi, C., Su, Y., Yin, Z., Zhang, S., Jin, L., Stoneking, M., Yang, J., Wang, K., Huang, H., Li, J., & Fan, S. (2025). An ancient regulatory variant of ACSF3 influences the coevolution of increased human height and basal metabolic rate via metabolic homeostasis. Cell Genomics, 100855, 100855. https://doi.org/10.1016/j.xgen.2025.100855